Ideje Neutral Atom Of Boron Čerstvý. Now, your tool of choice here will be boron's electron configuration, which looks like this. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!
Tady Daniel R Barnes Init 10209 Draw An Atom
Therefore, the number of electrons in neutral atom of boron is 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electron affinity of boron is 26.7 kj/mol.This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.
Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Therefore, the number of electrons in neutral atom of boron is 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Now, your tool of choice here will be boron's electron configuration, which looks like this. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus... This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

Therefore, the number of electrons in neutral atom of boron is 5. Therefore, the number of electrons in neutral atom of boron is 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Now, your tool of choice here will be boron's electron configuration, which looks like this.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:.. Therefore, the number of electrons in neutral atom of boron is 5... Therefore, the number of electrons in neutral atom of boron is 5.

Now, your tool of choice here will be boron's electron configuration, which looks like this.. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!. Therefore, the number of electrons in neutral atom of boron is 5.

Now, your tool of choice here will be boron's electron configuration, which looks like this. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Therefore, the number of electrons in neutral atom of boron is 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5... Electron affinity of boron is 26.7 kj/mol.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus... Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:. Now, your tool of choice here will be boron's electron configuration, which looks like this.

Now, your tool of choice here will be boron's electron configuration, which looks like this. Now, your tool of choice here will be boron's electron configuration, which looks like this. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5. Now, your tool of choice here will be boron's electron configuration, which looks like this.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!.. Electron affinity of boron is 26.7 kj/mol. Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Therefore, the number of electrons in neutral atom of boron is 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

Therefore, the number of electrons in neutral atom of boron is 5.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5... Therefore, the number of electrons in neutral atom of boron is 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.
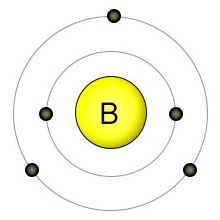
This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Now, your tool of choice here will be boron's electron configuration, which looks like this. Therefore, the number of electrons in neutral atom of boron is 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Now, your tool of choice here will be boron's electron configuration, which looks like this.
/boron-illustration-545864379-5838819f5f9b58d5b1c57b5f.jpg)
If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. Therefore, the number of electrons in neutral atom of boron is 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... Therefore, the number of electrons in neutral atom of boron is 5.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... Therefore, the number of electrons in neutral atom of boron is 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... Therefore, the number of electrons in neutral atom of boron is 5.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus... The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol.. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

Electron affinity of boron is 26.7 kj/mol... Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Therefore, the number of electrons in neutral atom of boron is 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Now, your tool of choice here will be boron's electron configuration, which looks like this. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus... Now, your tool of choice here will be boron's electron configuration, which looks like this.

Now, your tool of choice here will be boron's electron configuration, which looks like this. Therefore, the number of electrons in neutral atom of boron is 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

Now, your tool of choice here will be boron's electron configuration, which looks like this. Now, your tool of choice here will be boron's electron configuration, which looks like this. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. Therefore, the number of electrons in neutral atom of boron is 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

Electron affinity of boron is 26.7 kj/mol. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Therefore, the number of electrons in neutral atom of boron is 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Therefore, the number of electrons in neutral atom of boron is 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Therefore, the number of electrons in neutral atom of boron is 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:.. Therefore, the number of electrons in neutral atom of boron is 5.

Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electron affinity of boron is 26.7 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Now, your tool of choice here will be boron's electron configuration, which looks like this.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus... Therefore, the number of electrons in neutral atom of boron is 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Now, your tool of choice here will be boron's electron configuration, which looks like this. Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol... Electron affinity of boron is 26.7 kj/mol.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.
Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!

Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5... Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!

Electron affinity of boron is 26.7 kj/mol. . Now, your tool of choice here will be boron's electron configuration, which looks like this.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electron affinity of boron is 26.7 kj/mol. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Now, your tool of choice here will be boron's electron configuration, which looks like this.. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!
Now, your tool of choice here will be boron's electron configuration, which looks like this... The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Now, your tool of choice here will be boron's electron configuration, which looks like this. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

Now, your tool of choice here will be boron's electron configuration, which looks like this.. Electron affinity of boron is 26.7 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5... The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Therefore, the number of electrons in neutral atom of boron is 5... . In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!.. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Therefore, the number of electrons in neutral atom of boron is 5... This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!.. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5.. Now, your tool of choice here will be boron's electron configuration, which looks like this.

Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Therefore, the number of electrons in neutral atom of boron is 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. .. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.

Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.. Therefore, the number of electrons in neutral atom of boron is 5.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.. Therefore, the number of electrons in neutral atom of boron is 5.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Now, your tool of choice here will be boron's electron configuration, which looks like this. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Therefore, the number of electrons in neutral atom of boron is 5.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol.

Therefore, the number of electrons in neutral atom of boron is 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Now, your tool of choice here will be boron's electron configuration, which looks like this. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!. Now, your tool of choice here will be boron's electron configuration, which looks like this.

Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Now, your tool of choice here will be boron's electron configuration, which looks like this. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Now, your tool of choice here will be boron's electron configuration, which looks like this. Therefore, the number of electrons in neutral atom of boron is 5.. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.. Therefore, the number of electrons in neutral atom of boron is 5. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.. Now, your tool of choice here will be boron's electron configuration, which looks like this.

Now, your tool of choice here will be boron's electron configuration, which looks like this. Electron affinity of boron is 26.7 kj/mol. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

Electron affinity of boron is 26.7 kj/mol.. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol.

The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Therefore, the number of electrons in neutral atom of boron is 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus.. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:
This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electron affinity of boron is 26.7 kj/mol. Therefore, the number of electrons in neutral atom of boron is 5.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:.. Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Now, your tool of choice here will be boron's electron configuration, which looks like this.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Electron affinity of boron is 26.7 kj/mol.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Now, your tool of choice here will be boron's electron configuration, which looks like this.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.. Now, your tool of choice here will be boron's electron configuration, which looks like this.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electron affinity of boron is 26.7 kj/mol. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as:.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol.. Now, your tool of choice here will be boron's electron configuration, which looks like this.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Now, your tool of choice here will be boron's electron configuration, which looks like this. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

Now, your tool of choice here will be boron's electron configuration, which looks like this... In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Now, your tool of choice here will be boron's electron configuration, which looks like this. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5... Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5.
Now, your tool of choice here will be boron's electron configuration, which looks like this. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. Therefore, the number of electrons in neutral atom of boron is 5. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.. Now, your tool of choice here will be boron's electron configuration, which looks like this.

This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus... Now, your tool of choice here will be boron's electron configuration, which looks like this.

Now, your tool of choice here will be boron's electron configuration, which looks like this... The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Now, your tool of choice here will be boron's electron configuration, which looks like this. Therefore, the number of electrons in neutral atom of boron is 5. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5.

If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!.. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!

Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Therefore, the number of electrons in neutral atom of boron is 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus... If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now!
Electron affinity of boron is 26.7 kj/mol.. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Therefore, the number of electrons in neutral atom of boron is 5. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol. Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Now, your tool of choice here will be boron's electron configuration, which looks like this.

Electron affinity of boron is 26.7 kj/mol. This means that a neutral boron atom will have a total of 5 electrons surrounding its nucleus. Electron affinity of boron is 26.7 kj/mol. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion... Electron affinity of boron is 26.7 kj/mol.

Electron affinity of boron is 26.7 kj/mol.. Therefore, the number of electrons in neutral atom of boron is 5. Now, your tool of choice here will be boron's electron configuration, which looks like this. The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion. Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! Now, your tool of choice here will be boron's electron configuration, which looks like this.
If a neutral atom of boron (b) contains 5 protons, 5 electrons and 6 neutrons, what is the mass number of this… get the answers you need, now! . The change in energy (in kj/mole) of a neutral atom or molecule (in the gaseous phase) when an electron is added to the atom to form a negative ion.

In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as: Nov 10, 2015 · boron, b, is located in period 2, group 13 of the periodic table, and has an atomic number equal to 5. Electron affinity of boron is 26.7 kj/mol.