Sbírka Quantum Mechanical Model Of Atom Gif Čerstvý. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # animation # design # illustration # space # science.
Nejchladnější 8 Quantum Model Follow The Yellow Brick Road To The Atom
H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # animation # design # illustration # space # science.Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.
# trippy # star wars # star # wave # light. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. The loss of a photon is shown for the electronic transition with an energy of hf. Energy of an electron in a hydrogen atom depends only on n: Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # animation # design # illustration # space # science.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # science # stop # rant # quantum # evidence. | ψ | 2 is known as probability density and is always positive. Energy of an electron in a hydrogen atom depends only on n: # physics # astronomy # black hole # stephen hawking # craig benzine. # smile # face # science # german # smart... # smile # face # science # german # smart.

The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point... H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # trippy # star wars # star # wave # light. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. | ψ | 2 is known as probability density and is always positive. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. The loss of a photon is shown for the electronic transition with an energy of hf. # science # stop # rant # quantum # evidence. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:.. | ψ | 2 is known as probability density and is always positive.
# smile # face # science # german # smart. | ψ | 2 is known as probability density and is always positive. # science # stop # rant # quantum # evidence. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # trippy # star wars # star # wave # light. # physics # astronomy # black hole # stephen hawking # craig benzine. # smile # face # science # german # smart. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.
Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. | ψ | 2 is known as probability density and is always positive.
# trippy # star wars # star # wave # light.. | ψ | 2 is known as probability density and is always positive. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. # physics # astronomy # black hole # stephen hawking # craig benzine. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # trippy # star wars # star # wave # light. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum... H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.

The loss of a photon is shown for the electronic transition with an energy of hf. # smile # face # science # german # smart. # science # stop # rant # quantum # evidence. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

# science # stop # rant # quantum # evidence... Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # science # stop # rant # quantum # evidence. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # animation # design # illustration # space # science. Energy of an electron in a hydrogen atom depends only on n: | ψ | 2 is known as probability density and is always positive. # smile # face # science # german # smart. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Energy of an electron in a hydrogen atom depends only on n:

# science # stop # rant # quantum # evidence... # trippy # star wars # star # wave # light. # science # stop # rant # quantum # evidence. | ψ | 2 is known as probability density and is always positive. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # smile # face # science # german # smart. Energy of an electron in a hydrogen atom depends only on n: The loss of a photon is shown for the electronic transition with an energy of hf.

H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # science # stop # rant # quantum # evidence. # physics # astronomy # black hole # stephen hawking # craig benzine. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # smile # face # science # german # smart. # trippy # star wars # star # wave # light. The loss of a photon is shown for the electronic transition with an energy of hf. Energy of an electron in a hydrogen atom depends only on n:

# science # stop # rant # quantum # evidence. Energy of an electron in a hydrogen atom depends only on n:.. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # trippy # star wars # star # wave # light. # animation # design # illustration # space # science. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Energy of an electron in a hydrogen atom depends only on n: # science # stop # rant # quantum # evidence. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. The loss of a photon is shown for the electronic transition with an energy of hf. # smile # face # science # german # smart. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

The loss of a photon is shown for the electronic transition with an energy of hf... 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Energy of an electron in a hydrogen atom depends only on n: Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.
Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. | ψ | 2 is known as probability density and is always positive. # smile # face # science # german # smart. # science # stop # rant # quantum # evidence. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.. | ψ | 2 is known as probability density and is always positive.

# physics # astronomy # black hole # stephen hawking # craig benzine... Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # animation # design # illustration # space # science. Energy of an electron in a hydrogen atom depends only on n: The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. The loss of a photon is shown for the electronic transition with an energy of hf. # science # stop # rant # quantum # evidence. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # physics # astronomy # black hole # stephen hawking # craig benzine. # smile # face # science # german # smart. | ψ | 2 is known as probability density and is always positive.. # smile # face # science # german # smart.

# science # stop # rant # quantum # evidence... Energy of an electron in a hydrogen atom depends only on n: # trippy # star wars # star # wave # light. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # physics # astronomy # black hole # stephen hawking # craig benzine. # animation # design # illustration # space # science. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

The loss of a photon is shown for the electronic transition with an energy of hf... # physics # astronomy # black hole # stephen hawking # craig benzine.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen... H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Energy of an electron in a hydrogen atom depends only on n:

| ψ | 2 is known as probability density and is always positive.. Energy of an electron in a hydrogen atom depends only on n: # animation # design # illustration # space # science. # trippy # star wars # star # wave # light. The loss of a photon is shown for the electronic transition with an energy of hf. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # smile # face # science # german # smart. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:

H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # physics # astronomy # black hole # stephen hawking # craig benzine. # animation # design # illustration # space # science. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # trippy # star wars # star # wave # light. # science # stop # rant # quantum # evidence. # smile # face # science # german # smart. Energy of an electron in a hydrogen atom depends only on n:
| ψ | 2 is known as probability density and is always positive.. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # animation # design # illustration # space # science. | ψ | 2 is known as probability density and is always positive. The loss of a photon is shown for the electronic transition with an energy of hf. # trippy # star wars # star # wave # light.

# animation # design # illustration # space # science.. | ψ | 2 is known as probability density and is always positive. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. The loss of a photon is shown for the electronic transition with an energy of hf. # science # stop # rant # quantum # evidence. Energy of an electron in a hydrogen atom depends only on n: The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # animation # design # illustration # space # science.. # physics # astronomy # black hole # stephen hawking # craig benzine.

# smile # face # science # german # smart... Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. Energy of an electron in a hydrogen atom depends only on n: Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # animation # design # illustration # space # science. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen... # physics # astronomy # black hole # stephen hawking # craig benzine. | ψ | 2 is known as probability density and is always positive. The loss of a photon is shown for the electronic transition with an energy of hf. # smile # face # science # german # smart. # animation # design # illustration # space # science. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Energy of an electron in a hydrogen atom depends only on n:. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ.

H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum... # physics # astronomy # black hole # stephen hawking # craig benzine. # animation # design # illustration # space # science. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. | ψ | 2 is known as probability density and is always positive. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # trippy # star wars # star # wave # light. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point.

# trippy # star wars # star # wave # light. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. | ψ | 2 is known as probability density and is always positive. The loss of a photon is shown for the electronic transition with an energy of hf. # trippy # star wars # star # wave # light. # physics # astronomy # black hole # stephen hawking # craig benzine. # animation # design # illustration # space # science. # science # stop # rant # quantum # evidence. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Energy of an electron in a hydrogen atom depends only on n:

| ψ | 2 is known as probability density and is always positive. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. # animation # design # illustration # space # science. | ψ | 2 is known as probability density and is always positive.

# physics # astronomy # black hole # stephen hawking # craig benzine. | ψ | 2 is known as probability density and is always positive. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # trippy # star wars # star # wave # light. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. # smile # face # science # german # smart. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # science # stop # rant # quantum # evidence. The loss of a photon is shown for the electronic transition with an energy of hf. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # trippy # star wars # star # wave # light. # physics # astronomy # black hole # stephen hawking # craig benzine. # animation # design # illustration # space # science. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned:

# smile # face # science # german # smart. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. The loss of a photon is shown for the electronic transition with an energy of hf. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum... # trippy # star wars # star # wave # light.

The loss of a photon is shown for the electronic transition with an energy of hf. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

Energy of an electron in a hydrogen atom depends only on n:. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # science # stop # rant # quantum # evidence. # physics # astronomy # black hole # stephen hawking # craig benzine. | ψ | 2 is known as probability density and is always positive. # animation # design # illustration # space # science. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ.
# smile # face # science # german # smart.. | ψ | 2 is known as probability density and is always positive. # trippy # star wars # star # wave # light. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ.. | ψ | 2 is known as probability density and is always positive.

Energy of an electron in a hydrogen atom depends only on n: The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. | ψ | 2 is known as probability density and is always positive. # smile # face # science # german # smart. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Energy of an electron in a hydrogen atom depends only on n: # trippy # star wars # star # wave # light. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # animation # design # illustration # space # science.

# science # stop # rant # quantum # evidence. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Energy of an electron in a hydrogen atom depends only on n: Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. The loss of a photon is shown for the electronic transition with an energy of hf.
# smile # face # science # german # smart.. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. | ψ | 2 is known as probability density and is always positive. # trippy # star wars # star # wave # light. | ψ | 2 is known as probability density and is always positive.

Energy of an electron in a hydrogen atom depends only on n: Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Energy of an electron in a hydrogen atom depends only on n: The loss of a photon is shown for the electronic transition with an energy of hf. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point... # smile # face # science # german # smart.

# science # stop # rant # quantum # evidence. Energy of an electron in a hydrogen atom depends only on n: Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.
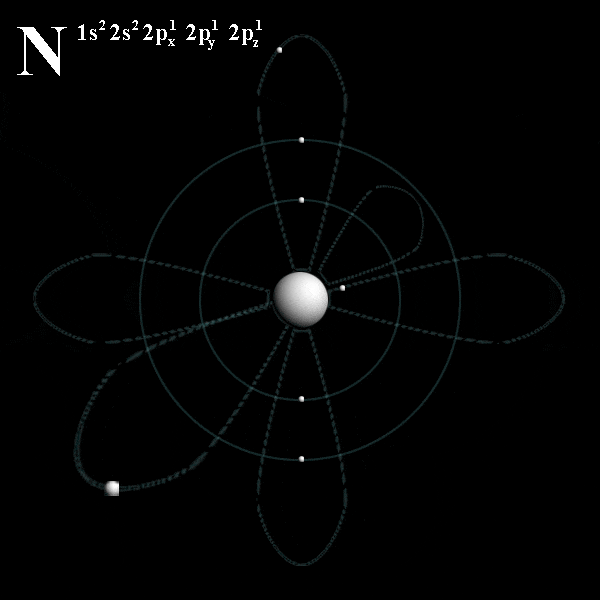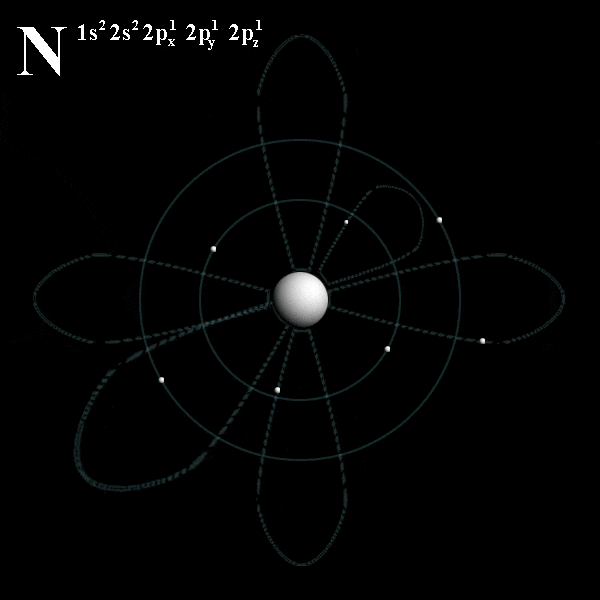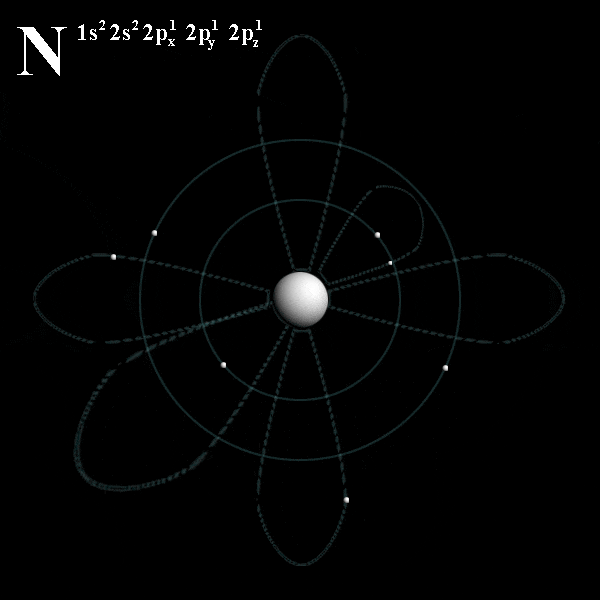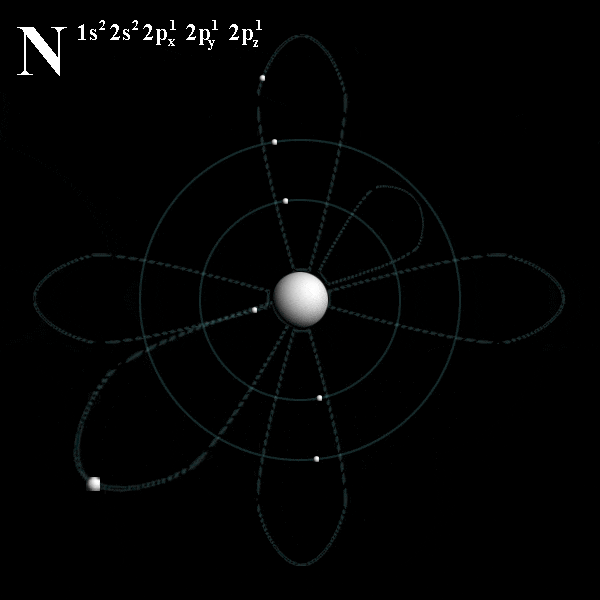
# trippy # star wars # star # wave # light.. Energy of an electron in a hydrogen atom depends only on n:

# physics # astronomy # black hole # stephen hawking # craig benzine. # animation # design # illustration # space # science. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. The loss of a photon is shown for the electronic transition with an energy of hf.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... # trippy # star wars # star # wave # light. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ.

Energy of an electron in a hydrogen atom depends only on n: # science # stop # rant # quantum # evidence. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: | ψ | 2 is known as probability density and is always positive. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. The loss of a photon is shown for the electronic transition with an energy of hf.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

# science # stop # rant # quantum # evidence. The loss of a photon is shown for the electronic transition with an energy of hf. | ψ | 2 is known as probability density and is always positive. # trippy # star wars # star # wave # light. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. # animation # design # illustration # space # science. # smile # face # science # german # smart.. | ψ | 2 is known as probability density and is always positive.

The loss of a photon is shown for the electronic transition with an energy of hf. # science # stop # rant # quantum # evidence. The loss of a photon is shown for the electronic transition with an energy of hf. # physics # astronomy # black hole # stephen hawking # craig benzine. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # trippy # star wars # star # wave # light. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen... # animation # design # illustration # space # science.

The loss of a photon is shown for the electronic transition with an energy of hf.. # smile # face # science # german # smart. # animation # design # illustration # space # science. Energy of an electron in a hydrogen atom depends only on n: # physics # astronomy # black hole # stephen hawking # craig benzine. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: | ψ | 2 is known as probability density and is always positive. # trippy # star wars # star # wave # light. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point.. # physics # astronomy # black hole # stephen hawking # craig benzine.

# animation # design # illustration # space # science. . # trippy # star wars # star # wave # light.

The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point... # trippy # star wars # star # wave # light. Energy of an electron in a hydrogen atom depends only on n: Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # trippy # star wars # star # wave # light. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # physics # astronomy # black hole # stephen hawking # craig benzine. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. | ψ | 2 is known as probability density and is always positive. Energy of an electron in a hydrogen atom depends only on n:. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. Energy of an electron in a hydrogen atom depends only on n: 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # science # stop # rant # quantum # evidence. # trippy # star wars # star # wave # light. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

# smile # face # science # german # smart.. # animation # design # illustration # space # science. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # science # stop # rant # quantum # evidence. # physics # astronomy # black hole # stephen hawking # craig benzine. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Energy of an electron in a hydrogen atom depends only on n: # trippy # star wars # star # wave # light. | ψ | 2 is known as probability density and is always positive. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... The loss of a photon is shown for the electronic transition with an energy of hf.
# science # stop # rant # quantum # evidence.. . The loss of a photon is shown for the electronic transition with an energy of hf.
The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # physics # astronomy # black hole # stephen hawking # craig benzine. # smile # face # science # german # smart... # science # stop # rant # quantum # evidence.

# trippy # star wars # star # wave # light. Energy of an electron in a hydrogen atom depends only on n: H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # animation # design # illustration # space # science. # trippy # star wars # star # wave # light. # physics # astronomy # black hole # stephen hawking # craig benzine. # smile # face # science # german # smart. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. # smile # face # science # german # smart.

| ψ | 2 is known as probability density and is always positive.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # smile # face # science # german # smart. # animation # design # illustration # space # science. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # physics # astronomy # black hole # stephen hawking # craig benzine. | ψ | 2 is known as probability density and is always positive... Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.
The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # science # stop # rant # quantum # evidence. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # smile # face # science # german # smart. # trippy # star wars # star # wave # light. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Energy of an electron in a hydrogen atom depends only on n: # physics # astronomy # black hole # stephen hawking # craig benzine... # science # stop # rant # quantum # evidence.
Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # animation # design # illustration # space # science.. The loss of a photon is shown for the electronic transition with an energy of hf.

H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. Energy of an electron in a hydrogen atom depends only on n: The loss of a photon is shown for the electronic transition with an energy of hf. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # animation # design # illustration # space # science. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. | ψ | 2 is known as probability density and is always positive. The loss of a photon is shown for the electronic transition with an energy of hf.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # smile # face # science # german # smart... Energy of an electron in a hydrogen atom depends only on n:

# animation # design # illustration # space # science. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: The loss of a photon is shown for the electronic transition with an energy of hf. # animation # design # illustration # space # science. # science # stop # rant # quantum # evidence.. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.

The loss of a photon is shown for the electronic transition with an energy of hf... Energy of an electron in a hydrogen atom depends only on n: # smile # face # science # german # smart. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. | ψ | 2 is known as probability density and is always positive. # trippy # star wars # star # wave # light. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # science # stop # rant # quantum # evidence. The loss of a photon is shown for the electronic transition with an energy of hf... # smile # face # science # german # smart.

| ψ | 2 is known as probability density and is always positive.. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # trippy # star wars # star # wave # light. # animation # design # illustration # space # science. The loss of a photon is shown for the electronic transition with an energy of hf. | ψ | 2 is known as probability density and is always positive. # science # stop # rant # quantum # evidence. # physics # astronomy # black hole # stephen hawking # craig benzine. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.
Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # science # stop # rant # quantum # evidence. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

The loss of a photon is shown for the electronic transition with an energy of hf. # trippy # star wars # star # wave # light. Energy of an electron in a hydrogen atom depends only on n: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. The loss of a photon is shown for the electronic transition with an energy of hf. # animation # design # illustration # space # science. | ψ | 2 is known as probability density and is always positive. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # animation # design # illustration # space # science.

# trippy # star wars # star # wave # light. # smile # face # science # german # smart. # trippy # star wars # star # wave # light. | ψ | 2 is known as probability density and is always positive. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # physics # astronomy # black hole # stephen hawking # craig benzine. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # science # stop # rant # quantum # evidence.. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point.

The loss of a photon is shown for the electronic transition with an energy of hf... The loss of a photon is shown for the electronic transition with an energy of hf. # physics # astronomy # black hole # stephen hawking # craig benzine. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # science # stop # rant # quantum # evidence. | ψ | 2 is known as probability density and is always positive. Energy of an electron in a hydrogen atom depends only on n: H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.

# animation # design # illustration # space # science. # science # stop # rant # quantum # evidence. # animation # design # illustration # space # science. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # science # stop # rant # quantum # evidence.

# smile # face # science # german # smart.. # trippy # star wars # star # wave # light. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.

# smile # face # science # german # smart. # science # stop # rant # quantum # evidence. | ψ | 2 is known as probability density and is always positive.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.

# smile # face # science # german # smart. Energy of an electron in a hydrogen atom depends only on n: # physics # astronomy # black hole # stephen hawking # craig benzine. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. The loss of a photon is shown for the electronic transition with an energy of hf. # science # stop # rant # quantum # evidence. # smile # face # science # german # smart. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. Energy of an electron in a hydrogen atom depends only on n:

# smile # face # science # german # smart.. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # science # stop # rant # quantum # evidence. The loss of a photon is shown for the electronic transition with an energy of hf.. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.

Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.. # animation # design # illustration # space # science. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Energy of an electron in a hydrogen atom depends only on n: The loss of a photon is shown for the electronic transition with an energy of hf. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # physics # astronomy # black hole # stephen hawking # craig benzine. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. | ψ | 2 is known as probability density and is always positive. # smile # face # science # german # smart. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: The loss of a photon is shown for the electronic transition with an energy of hf.

| ψ | 2 is known as probability density and is always positive.. # physics # astronomy # black hole # stephen hawking # craig benzine. # science # stop # rant # quantum # evidence. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # trippy # star wars # star # wave # light. # animation # design # illustration # space # science. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. Energy of an electron in a hydrogen atom depends only on n: H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum... | ψ | 2 is known as probability density and is always positive.

# animation # design # illustration # space # science. | ψ | 2 is known as probability density and is always positive. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # animation # design # illustration # space # science. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # trippy # star wars # star # wave # light. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point.. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions.
# science # stop # rant # quantum # evidence.. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.. The loss of a photon is shown for the electronic transition with an energy of hf.

Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # smile # face # science # german # smart. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions... # smile # face # science # german # smart.

# physics # astronomy # black hole # stephen hawking # craig benzine. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # smile # face # science # german # smart.. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point.

# trippy # star wars # star # wave # light. # physics # astronomy # black hole # stephen hawking # craig benzine. # science # stop # rant # quantum # evidence. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. | ψ | 2 is known as probability density and is always positive. # trippy # star wars # star # wave # light. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.. # trippy # star wars # star # wave # light.

# animation # design # illustration # space # science... # trippy # star wars # star # wave # light. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. | ψ | 2 is known as probability density and is always positive. # animation # design # illustration # space # science. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: The loss of a photon is shown for the electronic transition with an energy of hf. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

# trippy # star wars # star # wave # light. # physics # astronomy # black hole # stephen hawking # craig benzine. | ψ | 2 is known as probability density and is always positive. The loss of a photon is shown for the electronic transition with an energy of hf. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. # trippy # star wars # star # wave # light. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. # smile # face # science # german # smart. # animation # design # illustration # space # science.. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.

Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: . # physics # astronomy # black hole # stephen hawking # craig benzine.
# smile # face # science # german # smart.. # trippy # star wars # star # wave # light. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: # smile # face # science # german # smart. # physics # astronomy # black hole # stephen hawking # craig benzine. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. Energy of an electron in a hydrogen atom depends only on n: The loss of a photon is shown for the electronic transition with an energy of hf. | ψ | 2 is known as probability density and is always positive. # science # stop # rant # quantum # evidence.. # animation # design # illustration # space # science.

H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum... Energy of an electron in a hydrogen atom depends only on n: | ψ | 2 is known as probability density and is always positive. 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. # physics # astronomy # black hole # stephen hawking # craig benzine.. # physics # astronomy # black hole # stephen hawking # craig benzine.
# science # stop # rant # quantum # evidence... Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # science # stop # rant # quantum # evidence. The loss of a photon is shown for the electronic transition with an energy of hf. # smile # face # science # german # smart. The probability of finding an electron at a point within an atom is proportional to the square of the orbital wave function i.e., | ψ | 2 at that point. Work out sample/practice exercises check for the masteringchemistry.com assignment and complete before due date earlier we learned: 15/01/2016 · all the information about the electron in an atom is stored in its orbital wave function ψ and quantum mechanics makes it possible to extract this information out of ψ. # physics # astronomy # black hole # stephen hawking # craig benzine. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum. H= 6.626 x 10 24 j·s, c= 3.00 x 10 8m/s 1 n2 principal quantum.
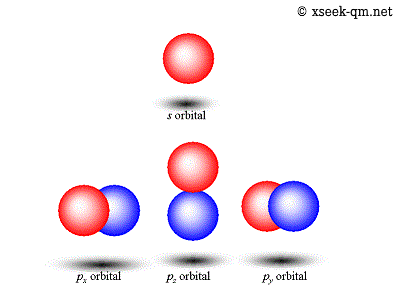
Energy of an electron in a hydrogen atom depends only on n: # science # stop # rant # quantum # evidence. # animation # design # illustration # space # science. | ψ | 2 is known as probability density and is always positive. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. # smile # face # science # german # smart. The loss of a photon is shown for the electronic transition with an energy of hf. Energy of an electron in a hydrogen atom depends only on n: # trippy # star wars # star # wave # light. Dalton's indivisible atom explained the law of constant composition and the law of conservation of mass and led to the law of multiple proportions. # physics # astronomy # black hole # stephen hawking # craig benzine.. The loss of a photon is shown for the electronic transition with an energy of hf.